BIO-BETTER/PEGylation
Bio-better/PEGylation
A biobetter is a biological that has been structurally and/or functionally altered to achieve an improved or a different clinical performance compared to an already approved original (innovator) biological product. Biobetters aim for the same target (e.g. receptor, enzyme) as the innovator product. Alterations used in the design of biobetters usually include chemical modification (e.g. PEGylation) or novel formulation (e.g. novel route of administration, modified release).
Improvements may result in longer half-life (enabling less frequent dosing), better efficacy and/or safety or reduced immunogenicity. Depending on their level of innovation and how well they meet a defined unmet medical need, biobetters may obtain patent and data exclusivity.
La tecnologia di PEGilazione può essere utilizzata per modificare composti biologici come citochine, frammenti di anticorpi, interleuchine, ormoni, oligonucleotidi e alcune proteine e peptidi.
La PEGilazione può essere efficace per migliorare la biodisponibilità, prolungare la circolazione sanguigna, massimizzare la farmacocinetica, abbasare l'immunogenicità e
diminuire la frequenza di dosaggio.
AQUILIBRIUM PHARMA fornisce PEG attivati ad alte prestazioni per i farmaci PEGylated dalla fase di sviluppo iniziale a quella commerciale grazie alla collaborazione con partner con esperienza ventenale nella produzione di metossipolietilenglicole di alta qualità, un materiale di partenza chiave del PEG attivato in campo farmaceutico.
I nostri PEG attivati hanno una stretta polidisperità e un basso contenuto di diolo con una vasta gamma di pesi molecolari, che vanno da 2kDa a 80kDa.
Biobetters are manufactured through chemical or molecular modifications of the originator product by functional changes that may include increased half-life, reduced toxicity, reduced immunogenicity, and enhanced pharmacokinetics and/or pharmacodynamics. This novel category of better biologics emerged over the last few years and gained industrial attention, mainly due to their reduced commercial risk, since they are patentable and worth higher prices due to their clinical advantages (Courtois et al., 2015; Lagassé et al., 2017). Biosimilars, on the other hand, are biologicals with equal efficacy as the originator drug at a reduced price. They are, however, not entitled to patent protection or data exclusivity (Kadam et al., 2016; Sandeep et al., 2016). Biosimilars aim to establish similarity to a known biological. Figure 1 depicts a general comparison between biosimilars and biobetters in terms of their properties and economical/regulatory aspects. In this review the main strategies for the development of ASNase biobetters will be explored by providing an updated overview and highlighting the inherent challenges and opportunities.
Biobetter of PegAspargase
PEGylation
Protein PEGylation is an important technique for the development of biopharmaceuticals with an improved quality profile, as prioritized by QbD principles (Turecek et al., 2016). Although PEGylation was firstly described in the late 1970s by Franck Davis and his colleagues (Abuchowski et al., 1977; Hoffman, 2016), new frontiers for this technology are now emerging, through advances in PEGylation chemistry and extension to a plethora of novel protein-based biopharmaceuticals (Ryu et al., 2012; Ginn et al., 2014; Swierczewska et al., 2015). Protein PEGylation consists in the attachment of polyethylene glycol (PEG) - an FDA approved polymer-to a protein-based drug, aiming at improving circulation half-life by reducing the rate of glomerular filtration (Ginn et al., 2014; Kolate et al., 2014; Turecek et al., 2016).
PEGylated proteins have arisen in the biopharmaceutical field as an endeavor to improve the clinical properties of protein-based biologics in terms of increasing circulation half-life without affecting the biological activity (Ginn et al., 2014; Turecek et al., 2016). Not only is the drug half-life boost witnessed an advantage of PEGylation, but also the enhancement of therapeutic efficacy of drugs, through several other beneficial effects, as described in Figure 3 (Harris and Chess, 2003; Ginn et al., 2014; Swierczewska et al., 2015; Turecek et al., 2016). PEGylation decreases protein aggregation by increasing its hydrophilicity, decreasing proteolytic degradation and recognition by anti-drug antibodies. However, PEGylation can negatively affect the in vitro activity of protein-based biologics. This effect can be offset in biological systems by the longer period of the drug circulation in blood vessels (Turecek et al., 2016).
Pegaspargase is a modified enzyme. It is a form of L-asparaginase that has undergone PEGylation. It is used as a cancer chemotherapy drug ("antineoplastic" or "cytotoxic") and is indicated for the treatment of acute lymphatic leukemia (ALL), non-Hodgkin's lymphoma and for the treatment of patients who have had a hypersensitivity reaction to a other form of asparaginase.
The originator product, Baxalta's Oncaspar (pegaspargase), was approved by the Food and Drug Administration (FDA) in the United States in February 1994 and by the European Medicines Agency (EMA) in January 2016.
Baxalta, a world leader in biotechnology, acquired Oncaspar in July 2015. The drug sold 87 million US dollars for Baxalta in 2015, with an annual historical turnover of around 1 billion US dollars. Oncaspar does not appear to be covered by any patent.
Current Trends in the Development of Novel Biobetter L-Asparaginases
Studies considering other kinds of bioconjugates have been reported aiming at the improvement of the ASNase quality profile (i.e., its pharmacokinetic and immunological properties) and the production of novel biobetter ASNases. Tabandeh and Aminlari (2009) investigated ASNase conjugation with oxidized inulin and improved pharmacokinetic and physicochemical characteristics were observed, such as resistance to trypsin digestion and higher thermal stability, longer half-life, better reusability after repeated freezing and wider optimum pH range than that of native ASNase. Furthermore, bioconjugation resulted in the decrease of antibody (IgG) titer and immunogenicity after repeated injections of rabbits when compared to the native ASNase. Zhang et al. (2005) prepared silk fibroin-ASNase bioconjugates that presented reduced immunogenicity and antigenicity, good residual activity (nearly 80%), increased thermal and storage stability, resistance to trypsin digestion and longer half-life (63 h) compared to the native enzyme (33 h). However, none of these new bioconjugates have undergone clinical trials yet.
In 2017, an Irish biopharmaceutical company has entered into a license agreement on the PASylation® Technology to develop a longer-acting ASNase. The PASylation is based on polypeptides composed of Pro, Ala and, alternatively, Ser (PAS), which, under physiological conditions, lack an ordered structure and form a random coil with surprisingly similar biophysical properties as PEG. Furthermore, due to the chemically inert methyl (Ala) and trimethylene (Pro) side groups, these polypeptides lack any side chain reactivity (Ahmadpour and Hosseinimehr, 2018; Gebauer and Skerra, 2018).
Two novel PEGylated ASNases are being developed: PEG-crisantaspase (Asparec®) and Calaspargase Pegol, as second-generation PEGylated drugs. In 2018, phase II and III clinical trials of PEG-crisantaspase were started and, as reported previously, their aim is to administer this biopharmaceutical as a second line therapy in cases of hypersensitivity to the E. coli ASNase. Recently, the FDA accepted the Biologics License Application (BLA) for a novel biobetter ASNase: Calaspargase Pegol. In this case, the SS linker of the reactive PEG employed for ASNase PEGylation was replaced by a succinimidyl carbamate linker, creating a more stable bioconjugate.
Biological and chemicals APIs
Aequilibrium Pharma continues to be a trusted partner for pharmaceutical companies, which rely on our complex and difficult-to-make Small Molecules Active Pharmaceutical Ingredients (APIs) for their formulation spanning of immunosuppressants, antifungals, antidiabetic and oncological etc.
We strive to build significant brand equity across our extensive customer base in both developed and emerging markets by leveraging our unique technological strengths in complex chemistry. We have the capability to synthesize complex steroids, complex APIs, deuterated APIs and Complex carbohydrates etc.
As one of the frontrunners in the contract manufacture of monoclonal antibodies (mAbs) and recombinant proteins Plant, Aeq pharma produces the essential ingredients for tomorrow’s life-saving medicines in four state-of-the-art cGMP multi-product facilities. Our customers range from international pharmaceutical companies and large biotechnology businesses to new start-ups. At AEQ PHARMA, we are prepared to meet the needs of the market and our clients to provide the best solution for your pipeline.
AEQ’s process R&D services range from vector construction and cell line development to full-scale manufacturing. These include a complete range of analytical services and regulatory support for clinical trials and in-market supply. . We have an expansive line of cutting edge Development Technologies available to enhance our expression process. From early protein target risk assessment programs to our continually upgraded and highly efficient GS Gene Expression , we are able to achieve higher titers, while improving speed to market, safety and costs.
We offer a full line development and manufacturing capabilities from DNA to BLA. In addition, we have full range of services for Antibody Drug Conjugate production. In conjunction with our Visp chemical facility, we can produce your mAb of interest and conjugate it to the drug of choice.
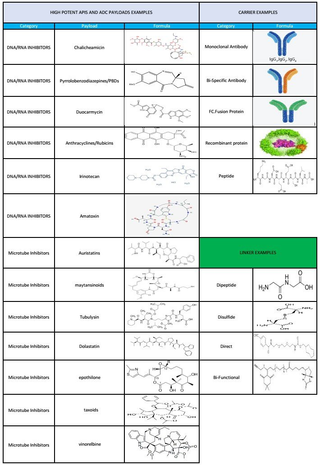
New Approaches to Conjugation
Through AEQ's R&D efforts, we have developed a novel linker for Lysine-based conjugation that demonstrates higher reactivity, better solubility and a more flexible range of conjugation temperatures. This linker offers the ability to conjugate to other molecules beyond IgGs. In addition, we have developed a unique payload chemistry to provide more homogenous drug loading for cysteine-based conjugation.
CYCLODEXTRIN
Subtitle
Alpha-Cyclodextrin
Beta-Cyclodextrin
Gamma-Cyclodextrin
Hexakis-(6-Mercapto-6-deoxy)-alpha-cyclodextrin
Hexakis-(6-Iodo-6-Deoxy)-alpha-Cyclodextrin
Hydroxypropyl-alpha-cyclodextrin;
HPACD
Hydroxypropyl-beta-cyclodextrin;
HPBCD
(2-Hydroxypropyl)-Alpha-Cyclodextrin;
HP-A-CD;
Hydroxypropyl-Gamma-cyclodextrin;
HP-G-CD;
Carboxymethyl-beta-cyclodextrin sodium salt;
CM-B-CD
Hydroxyethyl-Beta-Cyclodextrin;
HE-B-CD
Methyl-Beta-cyclodextrin;
Me-B-CD
Mono-(6-Amino-6-deoxy)-Beta-cyclodextrin
Heptakis(6-amino-6-deoxy)-beta-cyclodextrin
Mono-(6-Mercapto-6-deoxy)-Beta-cyclodextrin
Mono-(6-(tetraethylenepentamine)-6-deoxy)-beta-Cyclodextrin
Allyl-beta-cyclodextrin
Oligo(lactic acid)-beta-Cyclodextrin
Hydroxybutyl-beta-Cyclodextrin
Vanillin-Beta-cyclodextrin
Methnol-Hydroxypropyl-Beta-cyclodextrin
Salicylic Acid-hydroxypropyl-beta-cyclodextrin
Azelaic Acid-Beta-cyclodextrin
Nicotine-Beta-cyclodextrin
Customize modified cyclodextrins
MACROLID
Subtitle
Vancomycin hydrochloride
Teicoplanin
Tobramycin Sulfate
Tobramycin Base
Moxidectin
Daptomycin
Imiquimod
Docetaxel
Moxifloxacin hydrochloride
Cabazitaxel
Colistimethate sodium
Zinc bacitracin
Natamycin
Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer.
Human epidermal growth factor receptor (HER) 2 (HER2) is overexpressed in 20–30% of breast cancers. HER2 is a preferred target for treating HER2-positive breast cancer. Trastuzumab and Pertuzumab are two HER2-targeted monoclonal antibodies approved by the Food and Drug Administration (FDA) to use as adjuvant therapy in combination with docetaxel to treat metastatic HER2-positive breast cancer. Adding the monoclonal antibodies to treatment regimen has changed the paradigm for treatment of HER2-positive breast cancer. Despite improving outcomes, the percentage of the patients who benefit from the treatment is still low. Continued research and development of novel agents and strategies of drug combinations is needed. A thorough understanding of the molecular mechanisms underlying the action and synergism of trastuzumab and pertuzumab is essential for moving forward to achieve high efficacy in treating HER2-positive breast cancer.
This review examined and analyzed findings and hypotheses regarding the action and synergism of trastuzumab and pertuzumab and proposed a model of synergism based on available information.